
Karen T. Brown, MD
Dec 17, 2021 8:29 AM
By Michael Mozdy
Primary liver cancer is not a good diagnosis; in the United States, less than 50% of people survive 2 years after their hepatocellular carcinoma (HCC) diagnosis and 5-year survival is only 10%.1 Part of the problem is that liver cancer often develops in people who have underlying liver disease, in particular cirrhosis, typically related to alcohol or hepatitis. The cancer and cirrhosis are a one-two punch of devastation for the largest organ of the body and there is little hope outside of a full liver transplant.
Physicians have been trying for decades to provide better options for patients who either need to be treated while they wait for a transplant match or who don’t qualify for a transplant. Our section chief of Interventional Radiology, Karen Brown, MD, is one of those physicians. Her work has extended the lives of countless patients and continues to challenge the conventional wisdom about what treatments should be used
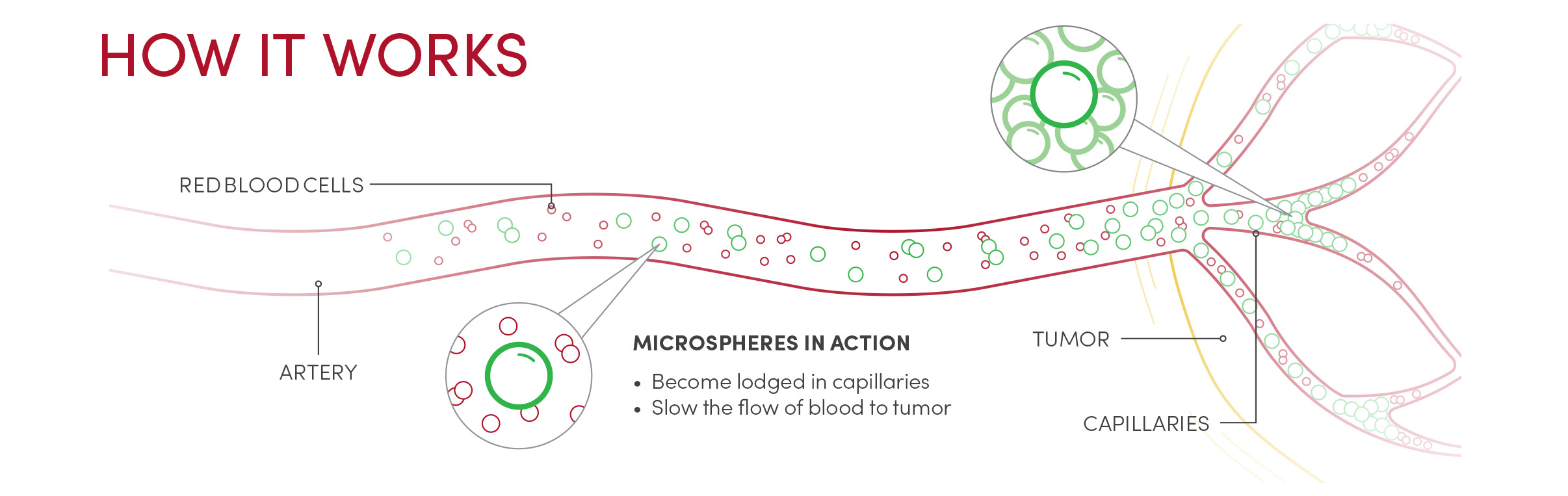
“In the 1980s,” she recalls, “a handful of large cancer centers in the U.S. were faced with a population of patients with HCC who had very limited options for treatment. Interventional radiologists in these centers started trying some new things.” Back then, there was no effective chemotherapy for HCC, which accounts for 80% of primary liver cancers. “It was hypothesized that maybe if the chemotherapy was retained in the tumor in higher concentrations for longer, rather than being administered broadly throughout the body at lower concentrations, the chemo would work better on the tumor with diminished systemic side effects” Brown explains. In order to do this, they started putting a chemotherapeutic slurry into the hepatic artery where it would travel into the tumors. The slurry included very small particles that would be carried into the tiny vessels feeding the tumors, causing these vessels to become blocked. This action of blocking a blood vessel is called embolization. This technique, called conventional trans-arterial chemoembolization, or cTACE, has two therapeutic effects: depriving tumors of oxygen (known to kill tumors) and theoretically maintaining higher concentrations of chemo at the site of the tumor.
In the first groups of patients studied, a number of chemotherapy drugs were mixed together with these particulates along with a third substance, lipiodol. “Lipiodol is iodinized poppy seed oil,” explains Brown. “It was known to be taken up by liver tumors and shows up on radiographs, so we can tell exactly where it is going.” The issue with the technique was that chemo agents are hydrophilic, while lipiodol is an oil. “You’re mixing it up like a salad dressing, administering it into the artery supplying the tumor and hoping that the chemo doesn’t wash away,” she says.
Some patients treated with cTACE lost their hair and developed low white blood cell counts – signs that the chemo was acting throughout the body, not just at the tumor sites. Pharmokinetic studies confirmed that most of the chemo was released into the bloodstream within 24 hours. Patients were seeing some benefit in terms of tumors shrinking, however. Was that short-lived dose of targeted chemotherapy enough to make the difference?
Finding Efficacy in Bland Embolics
Brown started practicing medicine at Memorial Sloan Kettering Hospital (MSKCC) in New York City in 1992 and, after reviewing the literature, suspected that something else was going on. Physicians elsewhere were comparing different chemotherapy cocktails only to find that there was absolutely no difference in response to treatment. “Most chemotherapeutic agents are toxic to the endothelium. In other words, since it will burn your skin it will also directly injure the cells lining the blood vessels it comes in contact with,” she relates. “My theory was that maybe all it’s doing is hurting the vascular walls, enhancing the effect of the particle embolics that were depriving the tumor of oxygen.”
In 1993, Brown and her colleagues at MSKCC started doing embolization without any chemotherapy agents, using the smallest embolic agents available in order to penetrate deep into the tumor vasculature. This method is known as “bland embolization.”
Brown’s work was advanced with the emergence of new embolization beads from manufacturers. At first, the only thing available were the particles made of PVA, or polyvinyl alcohol. “It was like chopping nuts on your counter,” she explains, “you end up with all different shapes and sizes.” The problem is that when you inject something like that, they can aggregate in the artery somewhere upstream before the spot you want them to go. “In order to kill a tumor by ischemia, one needs to penetrate into the small intra-tumoral blood vessels, otherwise blood flow can be recruited from other vessels around it,” she states.
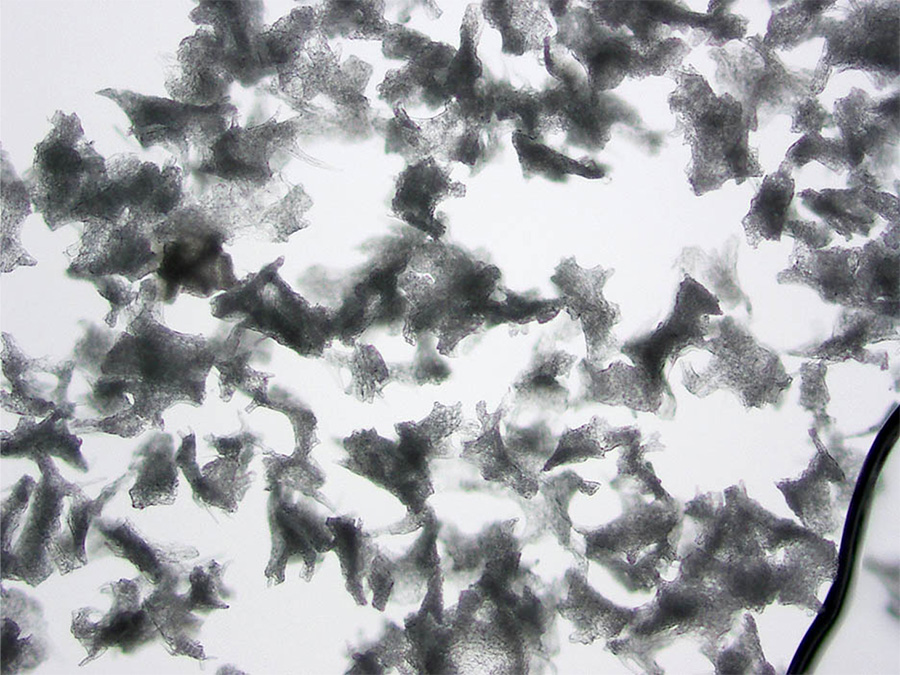
|
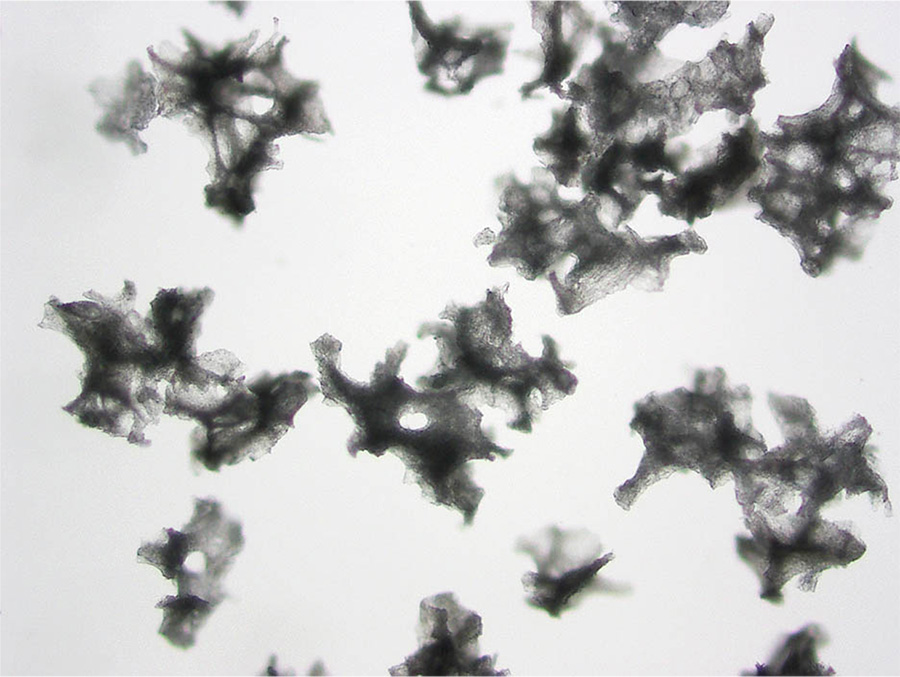
|
Photomicrographs of PVA from two different manufacturers, taken by Brown.
Around the year 2000, something new became available: a spherical embolic with a hydrogel coating that made the embolic “slippery” so it could be easily injected through a microcatheter and travel further in blood vessels. These spheres could be filtered through finer and finer screens to offer physicians narrow size ranges for their work. At first, they used spheres between 100-300 microns in diameter. For comparison, a red blood cell is just 10 microns across. The tighter calibrations available today result in spheres 40-60 microns in diameter, a size Brown prefers for single tumors smaller than 3 cm in diameter. If there are multiple and/or larger tumors, she might use larger spheres so that a fewer particles can fill the entire tumor volume to completely occlude the vessels.
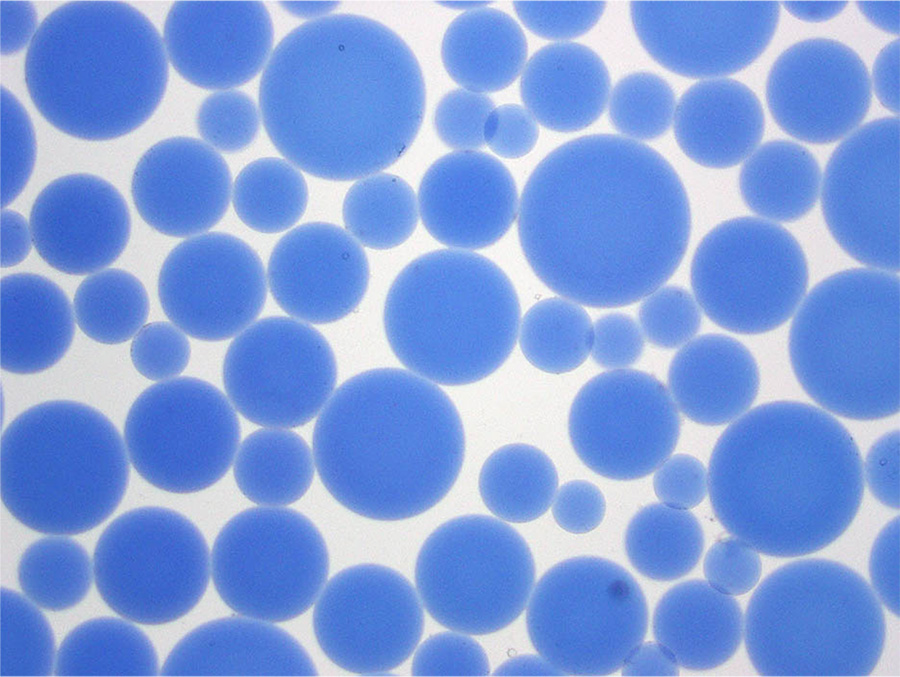
|
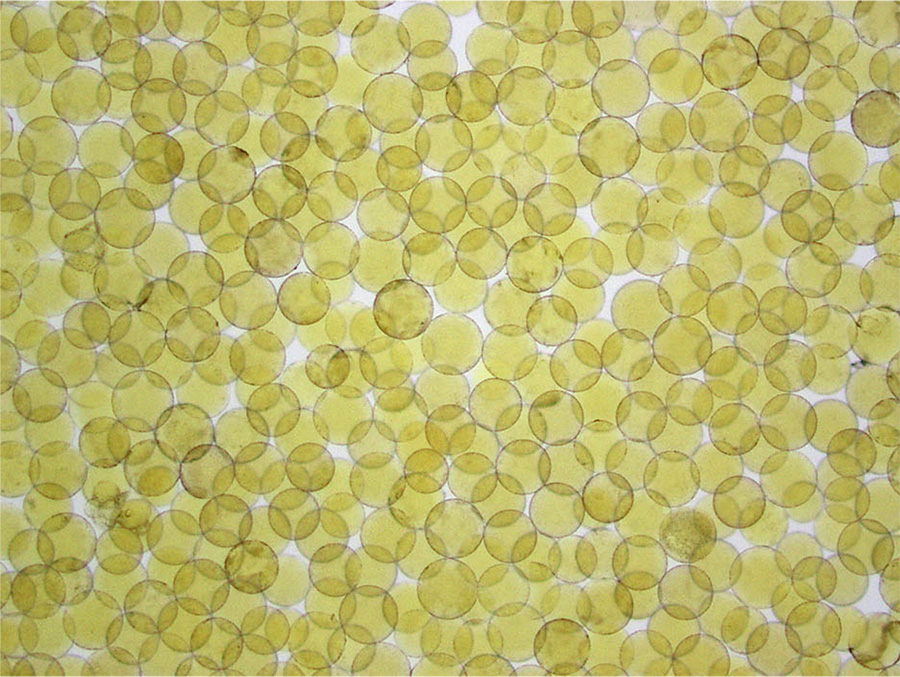
|
Photomicrograph of hydrophilic microspheres. Demonstrating the type of scientist she is, Brown wanted to confirm for herself the specs provided by the manufacturers and took these photomicrographs herself. On the left are microspheres sold in a 100-300 micron range and on the right are microspheres tightly calibrated to 100 microns.
In the early 2000s, Brown saw that other institutions were publishing data about patient outcomes following chemoembolization. She knew that they were having just as good of success if not better with the bland embolization technique in use at MSKCC. She embarked on a randomized clinical trial comparing bland and chemoembolization, something not common in the field of interventional radiology because so many of their techniques are new and experimental. They randomized 100 patients between 2008 and 2012 – half undergoing bland and half undergoing chemoembolization – and discovered that there was no significant difference in response to treatment weeks to months after the treatments. They used overall survival as a secondary endpoint (the number of patients was not high enough to provide the study with sufficient statistical power to generalize this survival rate for the entire population), and also found no significant difference between the techniques. This landmark paper was published in 2016 – the only randomized clinical trial of its kind in North America.
Comparing Studies and Costs
Brown’s paper is like most cancer clinical trials in that it includes data from the general population of people who were eligible for the treatment; which makes it unlike many interventional radiology papers that only report data on people who received the treatment. This means that if someone who was eligible is disqualified later because they develop some other problem – or if they die for a reason unrelated to the treatment – they are still included and the resulting data skews conservatively towards lower efficacy and shorter survival times. Even with these factors, no significant difference was found between bland embolization and chemoembolization.
Brown decided to look at the data in the way that interventional radiologists typically publish their data: only accounting for the patients who actually received the treatments. She found that the evidence pointed even more solidly toward bland embolization. Most heartening was the secondary endpoint, overall survival. Her study, albeit limited to 46 patients in each group, showed that those receiving bland embolization seemed to have a better survival probability over time, as the graph below shows.
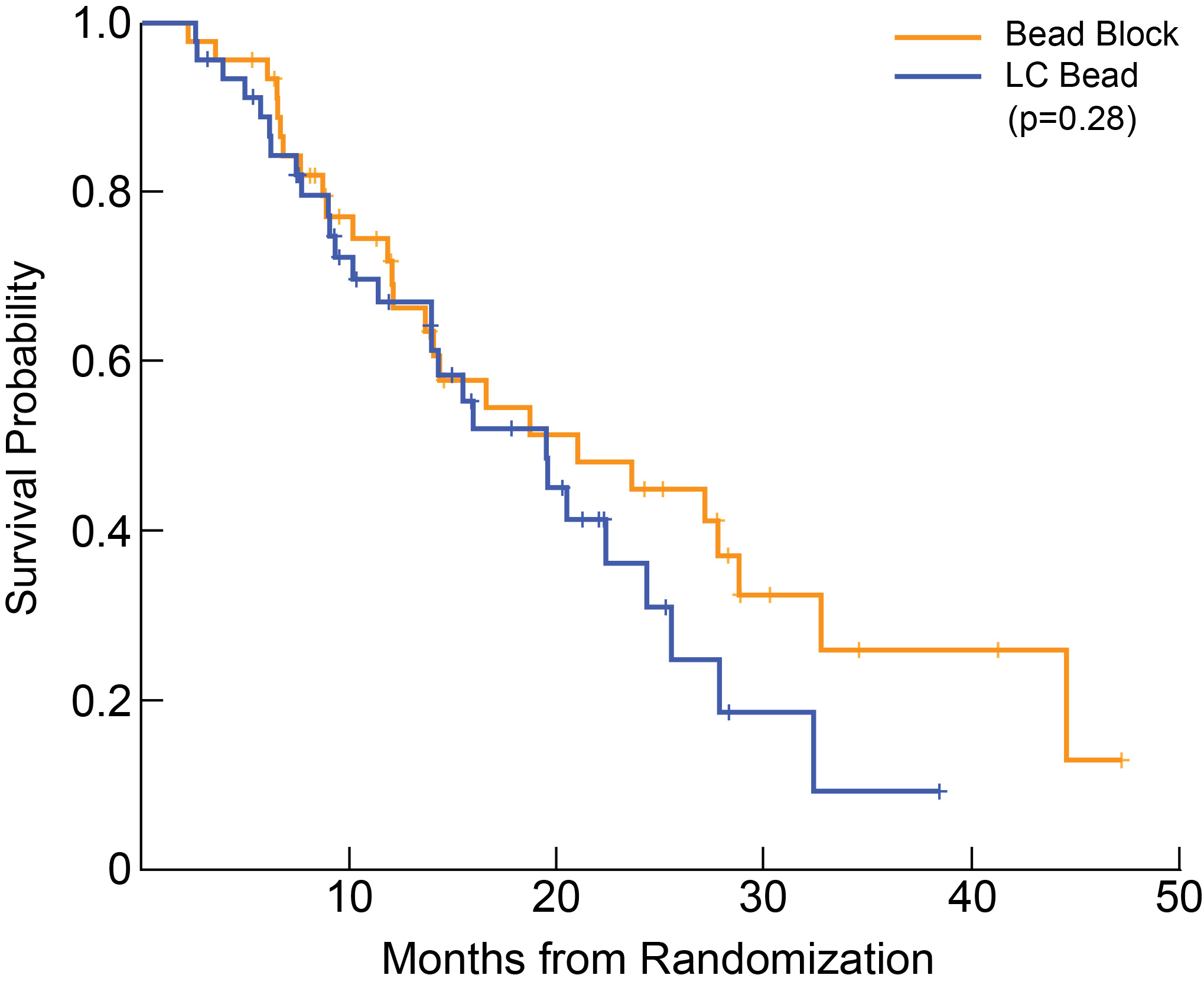
|
Median Overall Survival was 21.4 months for bland microspheres (95% CI 14.1 to N/A) and 20.8 months for microspheres loaded with chemotherapy agents (95% CI 14.0-27.9, p = 0.283).
Chemoembolization has progressed beyond the salad-dressing slurry days. There is now the option to use a bead that can be loaded with a chemotherapeutic agent in the pharmacy before coming to the surgical suite. But here’s the kicker: a bland spherical embolic costs $165 per vial while the chemo-loaded version costs $1,900 per vial. What’s more, the pharmacy loads a specific dose into the beads, and if those beads don’t do the work of fully filling the tumor volume, then the patient typically comes back for another treatment. Finally, the technique often used for chemoembolization is a “pruned tree” approach where the main vessel is left open while the smaller vessels that radiate from it are filled, but this is a subjective measure that may vary from physician to physician. Using bland embolization technique, the tumor is completely embolized on the first pass.
If the outcomes are the same, Brown wonders, why would a physician choose to use expensive, more complicated to administer and potentially hepato-toxic agents in a cancer patient when bland embolization is available?
Part of the reasoning may be that physicians and patients both tend to embrace a “guns blazing” approach that errs on the side of more powerful agents fighting the cancer. Another factor may be the conventional thinking that chemotherapy is simply a well-established, accepted and desirable treatment for cancer, and this is what many doctors have been trained to do.
Considering global medicine in particular – that is, medicine practiced in developing countries – a cheaper, safer-to-handle embolic with an easier protocol seems to be a no-brainer.
Radiotherapeutics Complicate the Equation
The other broadly accepted therapy for cancers when surgery isn’t an option is radiation therapy. In the late 1990s and early 2000s, physician researchers and manufacturers were able to create very small glass or resin beads loaded with a radioactive agent. The most widely known radioembolic is Yttrium-90, or Y-90. Y-90 emits beta-ray radiation, which travels a shorter distance than gamma rays or x-rays, and the spheres only emit radiation for about 10 days. A study in 2016 showed that Y-90 radioembolization resulted in significantly prolonged progression free survival compared to conventional chemoembolization (the salad-dressing technique).2 It seemed like a new player had emerged to be the best solution for HCC patients.
Indeed, Brown admits that Y-90 is a very good option for patients who are awaiting a liver transplant. “The workup for transplant alone takes 2-3 months,” she states, “and then you have to wait 3-18 months for a match. Meanwhile, if you have a liver tumor, you have to treat it or it could progress to the point where the patient no longer meets transplant criteria.” Since Y-90 radioembolization has a longer time-to-tumor progression, it seems to be the best option for those awaiting transplant.
However, Brown cautions against recommending it for all HCC patients. “Y-90 has been shown to result in deterioration of liver function,” she states. It’s a strong hammer to use on a vital organ, and there is only so much radiation the liver can take before the underlying cirrhosis progresses, sometimes to liver failure. For those who are not able to receive a transplant for any of a number of reasons, Y-90 may not be a good choice. “I’ve done bland embolization 13 times on some patients with other vascular tumors” – such as slow-growing neuroendocrine tumors in the liver – “it’s unlikely that one could administer Y-90 13 times without resulting in significant progression of cirrhosis beyond the first year or two of treatment,” she says.
And if you think the $1,900-per-vial price is high for chemoembolization, Y-90 checks in at a whopping $18,000 per dose! And a person might need more than one dose per treatment. This is a significant consideration for insurers, patients, and providers alike. Y-90 treatments are cost-saving in that they are able to be performed on an outpatient basis, saving money on the inpatient stay for bland and chemoembolizations (usually one night, around $5,000).
Brown believes that Y-90 treatments may be the best option for some patients, but certainly not all. The National Comprehensive Cancer Network (NCCN) guidelines for HCC patients were revised in 2016 to recommend all four minimally invasive trans-arterial therapies: bland, conventional chemoembolization, chemoembolization with drug loaded beads, and Y-90 radioembolization. Yet if you visit major cancer center websites from around the country, you rarely see bland embolization mentioned.
Brown would like to see that change, and her studies back her up.
It’s a case where we have to resist jumping at the “latest and greatest” techniques that are touted in the broader community before we study the science. “People clamor for science and then sometimes they just ignore it,” Brown laments. For HCC patients, this might mean reconsidering that expensive, toxic, and complicated therapy in favor of something less expensive, just as effective, non-toxic, and easier for physicians to apply.
1 Golabi P, Fazel S, Otgonsuren M, Sayiner M, Locklear CT, Younossi ZM. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine (Baltimore). 2017;96(9):e5904. doi:10.1097/MD.0000000000005904
2 Gordon A.C, et al. Gastroenterology 2016 Dec:151(6):1155-1163